Electrode potential transforms surfaces
Oxygen and chlorine evolution without noble metals
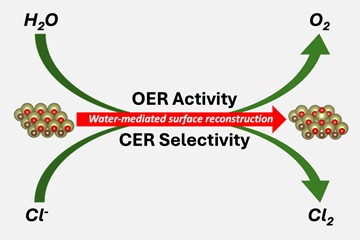
An applied electrode potential transforms the MXene surface: non-noble metal atoms migrate out and form 'SAC-like structures' – active centers for the evolution of gaseous oxygen or gaseous chlorine.
They are highly selective and can be easily separated from the reaction mixture: single-atom catalysts combine the advantages of homogeneous and heterogeneous catalysis. Until now, their production has generally been associated with precious metals that are anchored to a solid substrate. Researchers led by the University of Duisburg-Essen have now shown that such structures can also be formed electrochemically – independently and without noble metals. Their findings, published in the scientific journal JACS*, open up new avenues for a simpler, more sustainable production of catalytically active materials.
MXenes are a class of two-dimensional materials that were only discovered in 2011. Theoretical studies previously predicted that they would not be catalytically active in anodic processes. Researchers led by Prof. Dr. Kai S. Exner, head of the Department of Theoretical Catalysis and Electrochemistry at the University of Duisburg-Essen (UDE), have now disproved this theory using multiscale modeling.
The scientists discovered that when an electrode potential is applied, the MXene surface changes into a brush-like structure: atoms of non-noble metals migrate out and form so-called "SAC-like structures" (single atom catalyst-like). These catalysts mediate two important reactions, namely the oxygen evolution and chlorine evolution reactions.
The result is a material whose surface has catalytically active sites without the addition of precious metals. 'We concluded that MXenes behave similarly to enzymes in an electrochemical environment: by applying an electrode potential, their active sites are created directly in the process,' explains Exner.
The team was also able to show that the resulting SAC-like structures are selective: if water and chloride ions are in the reaction environment at the same time, only gaseous chlorine is formed. The formation of this base chemical is a key process in the chemical industry, which produces more than 70 million tons of gaseous chlorine (Cl2) per year. Cl2 is required for the production of pharmaceuticals, plastics, batteries, and for water treatment. However, when only water is available in the electrolyte, the active MXene surface facilitates the production of gaseous oxygen (O2) by means of oxygen evolution – an important step in the formation of green hydrogen in an electrolyzer.
This discovery can greatly simplify the production of single-atom catalysts. The elimination of expensive precious metals also reduces costs and dependencies.
The study also involved researchers from the University of Barcelona (Spain) and scientists from Ruhr Explores Solvation (RESOLV). RESOLV is a cluster of excellence of the University Alliance Ruhr.
*Journal of the American Chemical Society
https://www.uni-due.de/2024-12-17-catalysis-with-electrochemistry-instead-of-precious-metals
